|
Shenzhen Berry Dental Laboratory Co.,Ltd
|
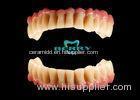
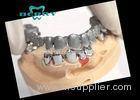
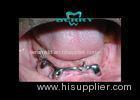
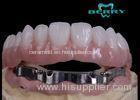
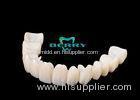
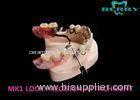
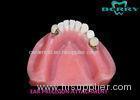
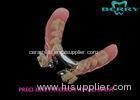
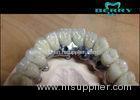
Implantable Telescope Crown restoring your perfect mouth state
| Place of Origin: | Zhejiang, China (Mainland) |
|
|
|
| Add to My Favorites | |
| HiSupplier Escrow |
Product Detail
Implantable Telescope Crown restoring your perfect mouth state
<h2 style="font-style:inh
Implantable Telescope Crown restoring your perfect mouth state
Material and Methods
Sixty-one implants were inserted in 7 adult patients (4 female and 3 male; age range 38 to 62 years). All patients were free of any medical conditions interfering with implant treatment. Five (71.43%) patients were smokers and 2 (28.58%) were nonsmokers. All patients had been referred from general dentists and were not previously treated for periodontal disease at the time of the first examination.
Each patient underwent a comprehensive dental and periodontal examination. Periodontal charting included documentation of probing depths, recessions, clinical attachment levels, bleeding on probing, tooth mobility, furcation involvement, and plaque scores. Periodontitis was diagnosed in the presence of more than 4 sites with clinical attachment loss exceeding 4 mm, radiographic evidence of alveolar bone loss, and bleeding on probing. Impressions for diagnostic casts were taken and a panoramic radiograph was obtained. Casts were mounted on a semi-adjustable articulator after face-bow transfer and check-bite registration. An occlusal analysis was performed, diagnostic wax-ups were prepared on the articulated casts, and restorative treatment needs determined. Once the restorative and periodontal treatment plans were established, radiographic and surgical guides were fabricated to facilitate implant placement. Table 1 shows the patient treatment plan and time schedule.
Periodontal treatment, including surgical treatment if necessary, had been performed previously on all patients.
Unless outlined differently, cylindrical screw implants with a large-grit sandblasted and acid-etched surface and either a 1.8-mm or a 2.8-mm smooth neck were used (ITI Straumann Standard Plus with a 1.8-mm smooth neck; ITI Straumann Standard with 2.8-mm smooth neck; Waldenburg, Switzerland; Table 2a and b). Implant size was determined based on assessment with a panoramic radiograph taken with a radiographic stent in place, and a clinical examination.
Standard medication for all cases included diclofenac (Voltaren; Novartis Pharma, Nürnberg, Germany), a nonsteroidal antiinflammatory drug, 100 mg once a day for 4 days; clindamycin (Ratiopharm, Ulm/Donautal, Germany), a systemic antibiotic, 600 mg once a day for 6 days; and 0.1% chlorhexidine rinses (Chlorhexamed Fluid, GlaxoSmithKline, Bühl, Germany) twice a day. Medication was administered starting 1 day before surgery.
Unless outlined differently for any individual case, procedures were performed following the protocols outlined below.
An intersulcular incision extending to the first adjacent tooth on each side was placed and a full-thickness flap was elevated.
Implant sites were prepared at 875 rpm using a 16:1 hand piece (Nouvag AG, Goldbach, Switzerland) and a microcomputer-controlled surgical micro motor (micro-dispenser model 7/8000, Nouvag).
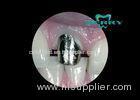
Implants were inserted and tightened to a torque of 35 N with a hand ratchet (model 046.119/046.049; Straumann).
The surgical site was covered with a resorbable bilayer membrane (BioGide, Geistlich Biomaterials, Wolhusen, Switzerland).
If tooth extraction was necessary in the area of implant placement, the indicated tooth was removed with as little surgical trauma as possible. Following curettage, the extraction socket was irrigated, and covered with a 25 × 30 mm, nonresorbable membrane (Cytoplast Regentex GBR-200, Oraltronics, Bremen, Germany). Membranes were removed after 4 weeks.
If necessary, sinus augmentation was performed following a previously described protocol.26 A 1:1 mixture of bovine allograft (0.25-1 mm, 0.25 g; BioOss spongiosa, Geistlich Biomaterials) and autogenous corticocancellous bone (harvested from the retromolar or chin area) was used as the graft material. The access window was covered with a resorbable barrier membrane (BioGide, Geistlich Biomaterials). The membrane was fixated with absorbable pins (Resor Pins, Geistlich Biomaterials).
Patients were instructed to avoid wearing any removable dentures for the first 2 weeks postoperatively. Postoperative follow-up visits were scheduled at 1, 4, and 7 weeks.
Implant placement was performed 4 to 6 months after the sinus augmentation surgery or simultaneously with the augmentation if the residual alveolar crest height exceeded 4 mm.
Supportive periodontal therapy was performed every 4 months. At each appointment, pocket depth (PD), clinical attachment level (AL), bleeding on probing (BOP), and plaque accumulation (PI) were recorded at 4 sites of each implant.
AL was defined as the distance in mm between the deepest point of the peri-implant area and the smooth neck section of the implant. Measurements were obtained by the use of a periodontal probe (KM0805, Hu-Friedy, Leimen, Germany).
Removal of soft and hard deposits around the implants and natural teeth, as well as irrigation of the peri-implant area with 0.1% chlorhexidine (GlaxoSmithKline), was performed at each visit; oral hygiene instructions were also given.
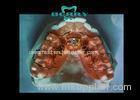
A 50-year-old man presented in the office for a complete oral rehabilitation. Clinical and radiographic evaluation revealed that none of the remaining teeth were salvageable for either periodontal or restorative reasons. Therefore, the treatment plan included the extraction of all remaining teeth; fabrication of a complete maxillary denture; and an implant retained, supported complete denture for the mandible, using telescopic crowns as attachments.
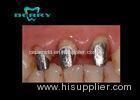
After the extraction of all remaining teeth, provisional complete dentures were delivered. Five months later, 6 ITI implants were placed in the mandible. Four months later a complete maxillary denture was delivered. An implant-supported complete mandibular denture using telescopic crowns as attachments was fabricated at the same time.
The 53-year-old woman was referred for periodontal and implant treatment by her general dentist. In the maxilla, only tooth No. 6 was remaining. This tooth was nonsalvageable.
Tooth No. 6 was extracted, and scaling and root planing was performed in the mandibular dentition. The periodontal condition appeared stable after the initial treatment phase. No further periodontal treatment other than regular supportive therapy was necessary.
Four months after tooth extraction, a bilateral sinus augmentation procedure was performed. After a healing period of 8 months, 8 ITI implants were placed in the maxilla. Six months after insertion, the implants were loaded with a telescopic crown retained removable denture. The denture was designed with a free palate (horseshoe-shape).
A 53-year-old woman presented in the office for a full mouth reconstruction. Clinical and radiographic examination revealed that none of the remaining teeth were salvageable.
It was decided to extract all the remaining teeth and to place implant retained overdentures.
Four months after extraction, sinus augmentation was performed bilaterally and 6 ITI implants each were placed in the maxilla and the mandible.
After a healing period of 6 months, implant retained overdentures, using telescopic crowns as attachments, were delivered. The maxillary overdenture was designed with a free palate (horseshoe-shape).
A 62-year-old man presented for an implant-supported full mouth reconstruction. All remaining teeth had to be extracted and provisional full dentures were delivered.
Six months later, 2 ITI implants were placed in the maxilla, and 6 ITI implants were placed in the mandible.